Overview regarding Patent Term Extension (PTE) in Japan
There are two main issues in Japan regarding the Patent Term Extension, and the following slides refer to one of them, Scope of Protection of Extended Patent Right.
Japanese Patent Law Article 67 defines the duration of patent rights:
- The duration of a patent right shall expire 20 years from the filing date (In the PCT case, 20 years from the international filing date).
- The duration may be extended not exceeding 5 years.
The objective of this article is: to restore the period during which a patented invention was unable to be worked because it was necessary to obtain an “disposition”, for example to assure the safety.
The “disposition” is defined as:
- Registration related to agricultural chemicals; and
- Regulatory Approval related to pharmaceuticals.
In Japan, the average patent term eroded to obtain the approval is about 8 years.
The basic limitations are:
- 5 years extension at the maximum
- Eligible products are: pharmaceuticals, in-vitro diagnostic reagents, regenerative medicine products, agrochemicals (not applied to intermediates catalysts, manufacturing apparatus).
New Drug Development Process in Japan
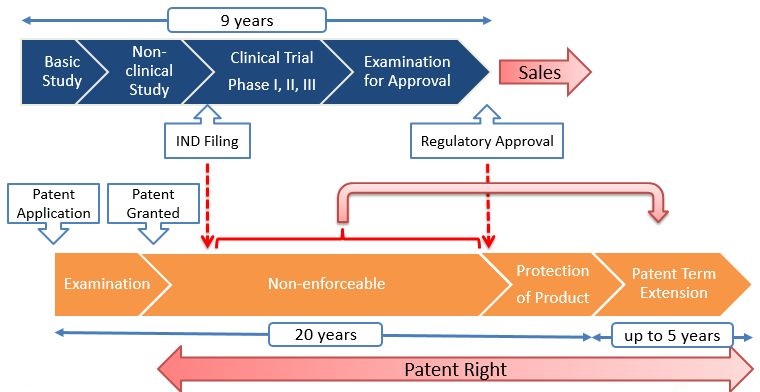
- Basic Study, about 2-3 years
- Non-clinical Study, about 3-5 years
- Clinical Trial, about 3-7 years
- Examination for Approval, about 1-2 years
- In total, it takes over 9 years on average.
The patent term can be extended for the term from the IND (“Investigational New Drug”) filing through the Regulatory Approval.
On average, the term of “Protection of Product” is about 6 years and the patent term extension is about 4 years.
While in the US, the “Protection of Product” is about 11 years and the patent term extension is about 3 years.
Basic Requirements for Patent Term Extension (PTE) in Japan
In order to obtain the Patent Term Extension, it is required to file an application for the extension.
The applicant should be the patent holder, and if the patent is owned jointly, the application should be filed by all of the holders.
The application should be filed before the original patent term expires, and, within 3 months of the regulatory approval.
The patent law defines some reasons for rejection, and about the “disposition”, it is required that:
- It was necessary to obtain the disposition; and
- The patentee or licensee obtained the disposition.
The patent term to be extended
- Starts: when the clinical trial is started or when the patent is registered, whichever is later; and
- Ends: when applicant receives regulatory approval.
The official fee for filing the application is JPY 74,000.
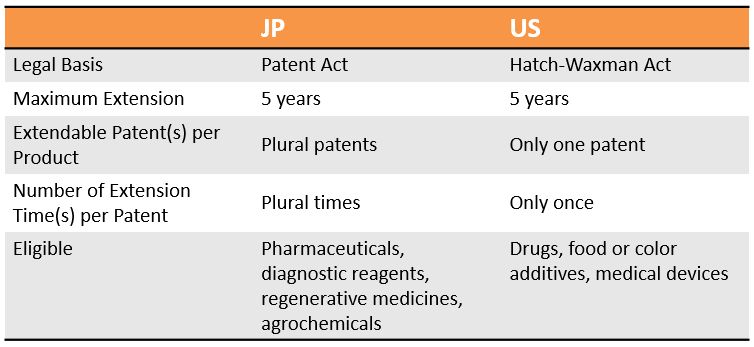
Comparison between JP and US PTE Practices
Two Main Issues regarding PTE in Japan
As to the Patent Term Extension, there are two main issues in Japan.
- whether it was necessary to obtain a disposition
- the scope of protection of extended patent right.
1. First Issue
As to the first issue, previously, when the pharmaceutical of a later disposition has the same “active ingredient” and “efficacy/effect” as an earlier pharmaceutical for which an earlier disposition was obtained, it was not possible to obtain the Patent Term Extension based on the later disposition.
However, in the Pacif case, the Supreme Court decided that: If the earlier pharmaceutical, for which the earlier disposition was already obtained, is not included in the technical scope of the patented invention, the patent term extension based on the later disposition is not denied.
After this case, the Examination Guidelines were revised in 2011.
Then, in the Bevacizumab case, the Supreme Court denied the JPO’s Guidelines and ruled that: Comparing the later disposition with the earlier disposition, if the implementation of the pharmaceutical covered by the earlier disposition includes that of the pharmaceutical covered by the later disposition (leading to the PTE application), it was “not necessary to obtain the present (new) disposition”.
And under the rule, the court decided in this specific case that: Even if the active ingredient and application of the prior and present pharmaceutical s are identical, the necessity of the later disposition should not be denied so long as dosage and administration are different.
After this case, the JPO revised the Examination Guidelines again in 2016, and this is the current version of the Examination Guidelines.
2. Second Issue
As to the second issue, Oxaliplatinum case, Debiopharm vs Towa Pharmaceutical, was issued in 2017 at the IP High Court. The Appeal to the Supreme Court was dismissed last year, and the High Court Decision became definite.
As to this case, please refer to the following descriptions.
After the big three court decisions, Pacif case, Bevacizumab case, and Oxaliplatinum case, the new Japanese operation regarding the extended patent rights has taken shape to some degree.
Overview of Oxaliplatinum Case
Main issues in this case were:
- Does Towa’s product fall within the scope of Debio’s patent?; and
- Is Debio’s extended patent right effective against Towa’s products?
Tokyo district court judged only on Issue II and said that: Debio’s extended patent right is not effective against Towa’s products
The IP High Court judged on both issues, and said that
- Regarding Issue II, Debio’s extended patent right is not effective against Towa’s products; and
- Regarding Issue I, Towa’s products do not fall within the scope of Debio’s patent.
In this case, two issues were contended, but, it is said that: If Issue I was first judged, it was not necessary to judge on Issue II. However, I think the IP High court dared to first refer to Issue II to set a guideline regarding the protection of extended patent right.
Overview of the Patent at Issue in Oxaliplatinum case
- Japanese Patent No.: 3547755
- Patent Holder: Debiopharm (Swiss)
- International Filing Date August 7, 1995
- Original Patent Term: August 7, 2015
- Extended Patent Term: January 29, 2020
- Claim 1: A pharmaceutically stable preparation of oxaliplatinum … consisting of a solution of oxaliptatinum … the oxaliplatinum content in the preparation being at least 95% …
- After receiving a first office action, the applicant filed an argument without amending the claims, and a patent was granted.
- 3 invalidation trials were filed against this patent, but this patent survived.
- Patent term was extended 7 times based on different dispositions.
Effect of Extended Patent Right in Japan
Japanese Patent Law Art. 68bis (Effect of patent right in the case of duration of patent right) defines that: Where the duration of a patent right is extended …, such patent right shall not be effective against any act other than the working of the patented invention for the product which was the subject of the disposition.
The fundamental policy is: The extended patent right is effective against the “working of the patented invention” for the “product” specified by the “ingredients, quantity, dosage, administration, efficacy, and effects” specified by a specific disposition.
However, such a policy would render the scope of the patent right too narrow, and so, the court decided that: Extended patent right is effective also against products which are substantially identical with said “product” as a medicine.
Interpretation of Substantially Identical
“Substantially Identical”?: Even if there is a part that differs from the subject product …, if said part is merely a slight difference or formal difference as a whole, it is reasonable to understand that the subject product is included in the products which are substantially identical with the product … as a medicine and falls within the scope of the extended patent right.
The IP High Court ruled 4 types where the products are recognized as being substantially identical as a medicine:
- where … a different ingredient (not the active ingredient) is partially added, converted, etc. based on well-known or commonly used art … in relation to a patented invention which is characterized only by the active ingredient of a medicine …;
- where … a different ingredient is partially added, converted, etc. based on well-known or commonly used art … in relation to a patented invention pertaining to stability or dosage form, etc. of a medicine with a publicly known active ingredient, and the products are recognized as being identical with each other in the technical features and function/effect in light of the content of the patented invention;
- … only a quantitatively meaningless difference … in terms of the “quantity” or “dosage and administration”; and
- … differ in the “quantity” … but are recognized as being identical with each other in consideration of “dosage and administration”.
Comparison of Debio and Towa Drug Products
In the Debio products, “ingredients” include only oxaliplatin and injectable water and do not include any other ingredients.
Meanwhile, in the Towa products, “ingredients” include concentrated glycerin as an additive (stabilizer), in addition to oxaliplatin and injectable water.
Therefore, Debio products and Towa products are literally different.
The products are different, but then, are they recognized as being “substantially identical”?
In this respect, the specification of the patent at issue describes: the purpose of the invention can be attained by using an “aqueous solution of oxaliplatinum that is free of any acidic or alkaline agent, buffer or other additive” and “this preparation is free of any other ingredients“.
Therefore, the court determined that: one of the technical features of the patented invention lies in the aqueous solution of oxaliplatinum free of any additive and that: the difference is not a slight difference or formal difference as a whole.
Accordingly, Towa products are not substantially identical with Debio products.
Key Takeaway regarding PTE in Japan
- the protection of extended patent right is effective not only against the “product” (drug) specified by the disposition, that is, “ingredients, quantity, dosage, administration, efficacy, and effects”, but also effective against the “substantially identical” product.
- the IP High Court ruled 4 types where the products are recognized as being “substantially identical”.
- Patent Term Extension practice in Japan:
- Plural patents can be extended based on one disposition, for example:
- material patent directed to an active ingredient of a drug;
- pharmaceutical patent directed to use of an active ingredient for a medical application approved; and
- process patent directed to a process of manufacturing an active ingredient.
- One patent can be extended multiple times based on plural dispositions directed to different products specified by ingredients, quantity, dosage, administration, and efficacy/effect of the disposition.
Recently, the technical importance of dosage form is growing by DDS (Drug delivery System).
Therefore, previous operation (where “the patent term extension is not allowed if the later disposition is the same as the prior disposition in terms of “active ingredient” and “efficacy/effect””) is no longer appropriate.
Now that the big three court decisions, which denied the previous operation, were made, I think the new Japanese operation has taken shape to some degree.
In practice, we are sometimes asked by foreign associates if the patents are eligible for the extension in Japan. So, when we receive an Allowance from the JPO regarding pharmaceutical patents, we ask our clients whether they wish to file an application for the extension.
When you file pharmaceutical patent applications in Japan, please consider whether it is possible to extend the patent term in Japan.
*We share a PPT material on “New Ruling on Scope of Protection of Extended Patent Rights in JAPAN”, on which Hiroshi Higuchi, a Managing Partner of Allegro IP, recently gave a presentation at the AIPLA Japan Committee’s Pre-Meeting in Tampa, FL
